Abstract
Introduction
In a phase 2 study designed to assess efficacy of extended treatment (tx) with KRd (carfilzomib [CFZ], lenalidomide [LEN], and dexamethasone [DEX]) plus autologous stem cell transplantation (ASCT), we reported high rates of deep responses, including high rates of stringent complete response (sCR) and minimum residual disease (MRD) negativity in newly diagnosed multiple myeloma (NDMM) patients (pts) after a median of 18 cycles (C) and 25.5 months (mo) of follow-up (f/u). In this analysis, we evaluated the impact of MRD negativity on progression-free survival (PFS).
Methods
The study enrolled ASCT-eligible pts with NDMM requiring tx per International Myeloma Working Group (IMWG) criteria with no age limitation. Pts received initial four 28-day cycles of KRd induction: CFZ IV 20/36 mg/m2 on days (D) 1, 2, 8, 9, 15, and 16 (20 mg/m2 on D1, 2 of C1 only); LEN PO D1-21 at 25 mg; DEX PO 40 mg/week followed by stem cell collection using G-CSF and plerixafor, melphalan 200 mg/m2, and ASCT. KRd consolidation (C5-8) used the same doses and schedule, except LEN 15 mg in C5 with the option to escalate to prior dose and DEX reduced to 20 mg weekly. After C8, pts received maintenance KRd for an additional 10 cycles using the same doses as in C8, except CFZ on D1, 2, 15, and 16 only. Single-agent LEN was recommended off-study after C18. Primary endpoint was rate of sCR at the end of C8, with MRD among secondary endpoints. Response rates and MRD were evaluated as per current IMWG criteria. MRD was evaluated by next-generation sequencing (NGS), using the immunoSEQ® Platform (Sequenta/Adaptive Inc.) with a sensitivity of 10-5-10-6 for MRD negativity, and multiparameter flow cytometry (MFC) with 10-4-10-5 sensitivity at landmark time points: after KRd consolidation (after C8), at the end of KRd tx (after C18), and then yearly. MRD-negative status required CR, as per current IMWG criteria.
Results
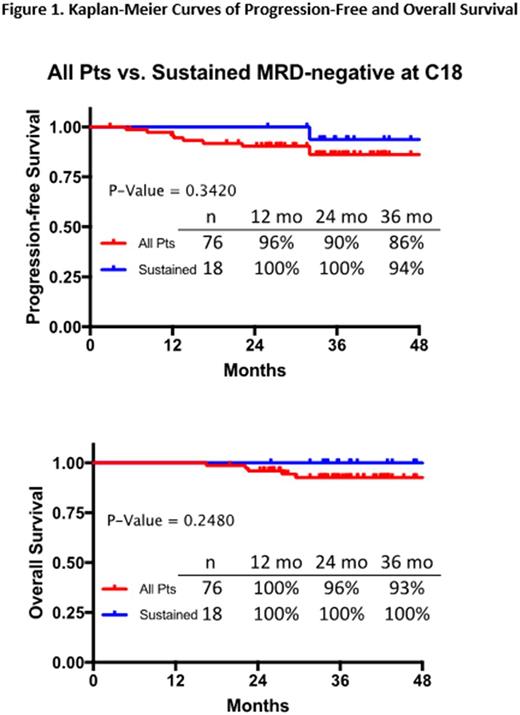
As of July 1, 2017, enrollment was completed (76 pts); 74 pts completed KRd induction, 72 ASCT, 70 consolidation, and 64 KRd maintenance with median f/u at cutoff date of 35.2 mo (range 2.9-53.0). Median age was 59 years (yr; range 40-76), 57% stage II/III of the International Staging System, and 36% high-risk cytogenetics as per IMWG criteria. For this analysis, efficacy data were available for 76 pts and MRD data from ≥1 landmark time point for 46 (by NGS) and 40 pts (by MFC). On intent-to-treat, the rate of ≥very good partial response was achieved in 91%, ≥CR in 78%, and sCR in 75% for all enrolled pts. At the end of C8, MRD negativity by NGS combined with ≥CR was observed in 67% (n=36) (including 10 of 13 pts with high-risk disease) and at the end of C18 in 72% (n=32) (including 7 of 10 high-risk pts). As expected, MRD rates were slightly higher by MFC at 95% (n=37) and 96% (n=27). Paired MRD results for the end of C8 and C18 landmarks were available for 34 of these pts by NGS and for 31 pts by MFC. At the end of C18, MRD negativity was sustained for 91% of MRD-negative pts by NGS and for 96% by MFC. At the cutoff date, 30 pts completed 1-yr LEN maintenance, which followed C18 of KRd tx, with MRD results available for 17 pts by NGS and 21 pts by MFC, with MRD-negative rates of 82% and 90%, respectively. Paired MRD results for the end of C18 of KRd tx and 1-yr LEN maintenance landmarks were available for 17 of these pts by NGS and for 20 pts by MFC. MRD-negative results were sustained in 93% of pts by NGS and 94% of pts by MFC. After median f/u of 35.2 mo, 3-yr PFS for pts with sustained MRD at the end of C18 by NGS was 94%, and for all pts (n=76) 86%. Overall survival (OS) rates at 3 yrs for sustained MRD-negative disease by NGS were 100%, and for all pts 93% (Fig. 1). In a subset of pts with high-risk disease (n=27), 3-yr PFS and OS rates were 81% and 87%, respectively. Updated results, including a larger sample of MRD data, will be presented at the meeting, with an increasing number of pts completing respective landmarks of tx and MRD evaluations.
Conclusions
These results show that extended KRd tx with incorporated ASCT results in high rates of deep responses, including high rates of MRD-negative disease. The achievement of high rates of sustained MRD-negative status correlates with high rates of 3-yr PFS and OS in overall and high-risk pt populations. These observations provide rationale for using sustained MRD-negative rates as a reliable predictor of improved tx outcomes for the entire pt population, which will require validation in ongoing and planned randomized trials.
Jakubowiak: Amgen Inc., BMS, Celgene, Janssen, Karypharm, Millennium-Takeda, Sanofi, SkylineDX: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; University of Chicago: Employment. Raje: Millenium: Consultancy; Celgene: Consultancy; Onyx: Consultancy; Amgen: Consultancy. Vij: Bristol-Meyers-Squibb: Honoraria; Amgen: Honoraria, Research Funding; Celgene: Honoraria; Jazz: Honoraria; Janssen: Honoraria; Takeda: Honoraria, Research Funding; Abbvie: Honoraria; Konypharma: Honoraria. Reece: Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Research Funding; Otsuka: Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Bristol-Meyers Squibb: Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Berdeja: Teva: Research Funding; Takeda: Research Funding; Amgen: Research Funding; Novartis: Research Funding; Janssen: Research Funding; Curis: Research Funding; Constellation: Research Funding; BMS: Research Funding; Bluebird: Research Funding; Celgene: Research Funding; Vivolux: Research Funding; Abbvie: Research Funding. Stephens: TG Therapeutics: Employment, Equity Ownership. Rosenbaum: Celgene: Honoraria. Richardson: Celgene: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Oncopeptides AB: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Severson: University of Chicago: Employment. McIver: The University of Chicago Biological Sciences: Employment. Wolfe: University of Chicago Medicine: Employment. Johnson: The University of Chicago >> BSD MED - Hematology and Oncology Research Staff: Employment. Dytfeld: Amgen: Consultancy; Celgene: Consultancy; Janssen: Consultancy. Turowski: University of Chicago: Employment. Hycner: University of Chicago: Employment. Zimmerman: Abbvie: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal